
Modules
Quality Control
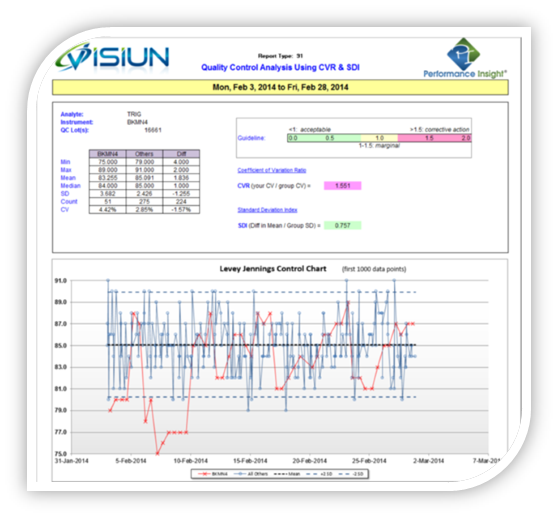
The Quality Control Module supports development of the CMS/CLIA prescribed Individualized Quality Control Plan (IQCP) based on CLSI EP23 guidelines. Best practices are identified using sigma ratings of instruments for each analyte to determine the appropriate number and frequency of QC samples to run. With real-time assessment of instrument performance, users can now know immediately if an instrument problem occurs. Access to this type of reporting ensures QC practices are properly implemented so labs can avoid repeat testing, unnecessary follow-up testing, and misdiagnoses.
Sigma Level Performance of IVD Clinical Equipment
Visiun now provides sigma level performance of IVD clinical equipment to assist laboratories with their vendor selection. Comprehensive sigma metrics cover over 250 analytes representing all of clinical pathology including Chemistry/Heme/Coag/UA. Visiun covers real-world conditions reflecting day-to-day operations to ensure performance and efficiency is measured accurately from all vendors and instrument models.
Features
- Supports real-time assessment of instrument performance
- Identifies best practices using sigma ratings of instruments for each analyte
- Analysis using either CVR & SDI or a standards analytical null hypothesis theory
- Sigma level performance of IVD clinical equipment
Benefits
- Instead of relying on peer comparison reports after an instrument problem occurs, labs immediately know if their instruments are not reporting in line with other analyzers
- Determines the appropriate number and frequency of QC samples to run, in some cases saving tens of thousands of dollars per year
- Provides two different approaches to quickly determine if any instruments are reporting differently than the others


